664
Views & Citations10
Likes & Shares
Background: Semaphorin 4D is a glycoprotein which was found to be highly expressed in many cancers, but its detailed pathological and prognostic role in HNSCC is still controversial. Homeobox (HOX) genes which code for many transcription factors which were aberrantly expressed in HNSCC tissues, like HOXB9 but, there is minimal research on the relationships between HOXB9 and HNSCC and there is a lack of clarity about its importance in HNSCC carcinogenesis.
Therefore we aimed in the current study to explore the expression of Semaphorin 4D and HoxB9 in malignant tissues which were retrieved from HNSCC patients then to correlate our findings with clinical data and pathological criteria of patients.
Methods: Semaphorin 4D and HoxB9 expression was evaluated in malignant tissues which were retrieved from HNSCC patients using immunohistochemistry then correlate their expression with clinical data and pathological criteria of patients.
Results: High Semaphorin 4D and HoxB9 expression was correlated with higher grade (p=0.043 and 0.039 respectively), presence of lymph node metastases (p=0.006 and 0.023, respectively), advanced stage of the tumor (p=0.002 and 0.015, respectively), distant metastases (p=0.025 and 0.030 respectively). There is a significant positive association between Semaphorin 4D and HoxB9 expression Phi: phi coefficient=+0.905 (p<0.001).
Conclusion: Semaphorin 4D and HoxB9 overexpression are markers of poor prognosis in HNSCC patients.
Keywords: Semaphorin 4D, HoxB9, HNSCC, Immunohistochemistry, Prognosis
INTRODUCTION
Head and neck squamous cell carcinomas (HNSCCs) are carcinomas which originate from the oral cavity, pharynx, larynx, nose and para-nasal sinuses [1]. They are considered the 6th commonest malignancy worldwide [2]. The nearby vital structures limits the radicalism of excision of such malignancies, moreover, patients could suffer from multiple primaries or have cancer recurrence with a 6 months average survival rate for advanced or recurrent cases [3,4]. Hence, there is a real need to find novel therapeutic intervention targets and new prognostic biomarkers in HNSCC [5]. Semaphorins are members of a large family of proteins which were discovered in the nervous system and they are involved in the maintenance of a correct neuronal network [6]. Semaphorin 4D is a glycoprotein which is a member of group IV semaphorin family and it could be present in a membrane bound or a secreted form [7]. It has a role in many physiological and pathological processes like angiogenesis and bone [8]. Semaphorin 4D was found to be highly expressed in many cancers [1,9], but its detailed pathological and prognostic role in HNSCC is still controversial.
Therefore we aimed in the current study to explore the expression of Semaphorin 4D and HoxB9 in malignant tissues which were retrieved from HNSCC patients then to correlate our findings with clinical data and pathological criteria of patients.
MATERIALS AND METHODS
This is a retrospective cohort study; we have included sections from fifty paraffin blocks which are retrieved from fifty HNSCC cases that were retrospectively examined. All cases were surgically managed at the Department of General Surgery, Department, Faculty of Medicine, Zagazig University Hospital in the period from March 2014 and March 2018. All patients’ data were collected from patients’ files and the diagnosis was pathologically confirmed to be HNSCC in pathology department, Faculty of Medicine Zagazig University and reviewed in Pathology Department Faculty of Medicine, Beni Suif University. No patient had received chemotherapy or radiotherapy before surgery. The study was approved by the ethics committee of Faculty of Medicine, Zagazig University.
Immunohistochemistry
Sections from the fifty paraffin blocks were deparaffinized then autoclaved in 0.2% citrate buffer for fifteen minutes for antigen retrieval. All sections were incubated with 3% hydrogen peroxide for half an hour to block endogenous peroxidase activity. Sections were incubated with primary rabbit polyclonal anti-human Semaphorin 4D (1:200, Abcam, Cambridge, MA, USA) anti-HOXB9 (1:400, Sigma Aldrich, Poole, UK) overnight at 4°C. The specimens were incubated with the primary antibodies overnight at 4˚C, followed by 3 washes with buffered saline, and then treated with a streptoavidin-biotin complex for 60 min at a dilution of 1:100. The immunoreaction was assessed using a 3,3'-diaminobenzidine (DAB) substrate-chromogen solution. Finally, the sections were immersed in both ethanol and xylene bath and then mounted for examination. Sections from colon cancer and submandibular salivary gland were used as positive controls for Semaphorin 4D and HoxB9, respectively.
Evaluation of the Immunohistochemical staining
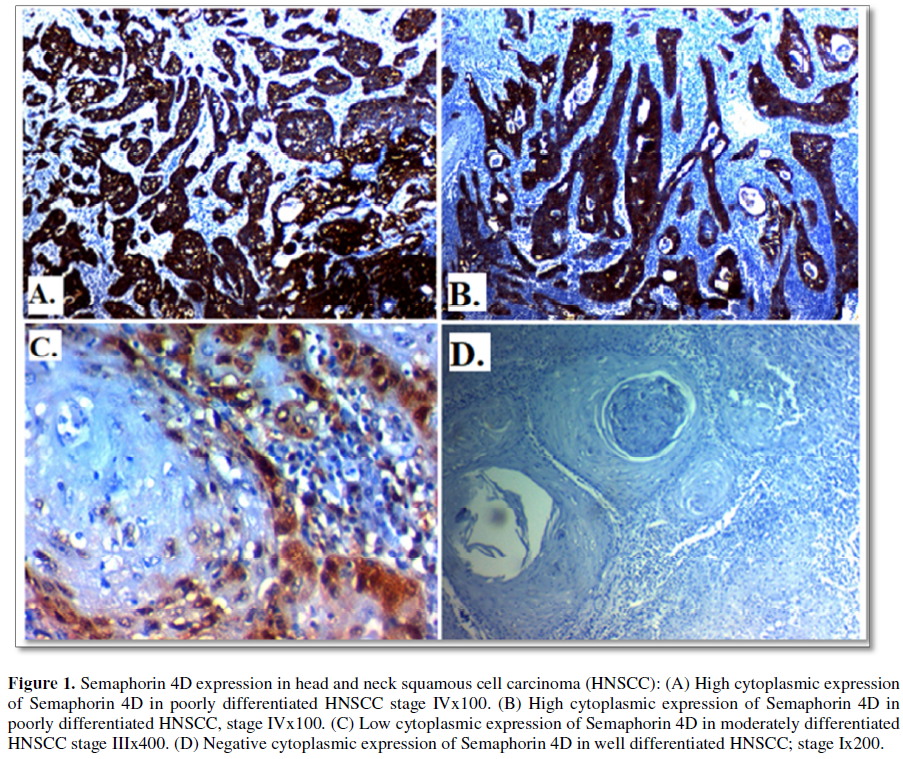
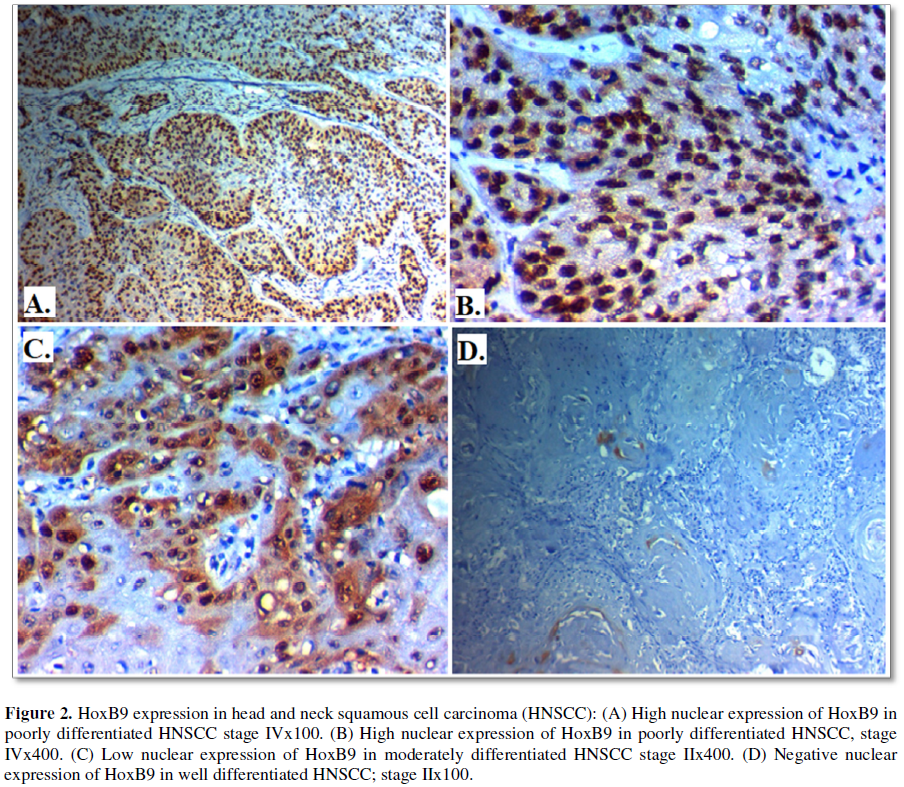
Cytoplasmic immunoreactivity for Semaphorin 4D and nuclear immunoreactivity for HOXB9 were evaluated according to both the extent and intensity of staining; the extent of positive tumor cells were scored as follows: 0: none; 1: <10%; 2: 10-50%; and 3: >50%, while the staining intensity were scored as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. We multiplied both to give final stain score from 0-9. Tumor cells with a score of ≥ 5 of staining were classified as high expression and tumor cells with a score of less than 5 were classified as low-expression group [1,9].
STATISTICAL ANALYSIS
Statistics were performed using SPSS 22.0 for windows (Chicago, IL, USA) and MedCalc windows (Ostend, Belgium). Percent of categorical variables were compared by using Chi-square test or Fisher's exact test when was appropriate. Continuous variables were expressed as the mean ± SD and median (range), and the categorical variables were expressed as a number (percentage). Strength of relationship between Semaphorin 4D and HoxB9 expression were determined by computing phi coefficient with (+) sign was indicator for direct relationship and (-) sign was indicator for inverse relationship. A p-value<0.05 was considered significant.
RESULTS
Patient characteristics
Fifty samples retrieved from HNSCC patients. The demographic data of the 50 patients were summarized in Table 1. We have included 38 (76%) males and 12 (24%) females.
Immunohistochemical results
Semaphorin 4D immuno-expression and its correlation clinicopathological features of HNSCC patients: High Semaphorin 4D expression was found in 35(70%) of cases of HNSCC and its expression was significantly positively correlated with higher grade (p=0.043), presence of lymph node metastases (p=0.006), advanced stage of the tumor (p=0.002), distant metastases (p=0.025), presence of adjacent foci of CIS (p=0.26), PNI (p=0.023). No significant correlation was found between Semaphorin 4D expression with primary site of the tumor, age or sex of patients or previous history of smoking (Table 2 and Figure 1).
HOXB9 immuno-expression and its correlation clinicopathological features of HNSCC patients: High HOXB9 was found in 30 (60%) of cases of HNSCC and its expression was significantly positively correlated with higher grade (p=0.039), distant metastases (p=0.030), advanced stage of the tumor (p=0.015), presence of lymph node (p=0.023), LVI (p=0.033) and higher incidence of P16 positivity (p=0.024), No significant association was found between HOXB9 expression with primary location of the tumor, age or sex of the patients, presence of adjacent foci of CIS or history of smoking (Table 3 and Figure 2).
There is a significant positive association between Semaphorin 4D and HoxB9 expression. Phi: phi coefficient=+0.905 (p<0.001).
DISCUSSION
The molecular pathogenesis of HNSCC is still poorly understood and despite recent advances in the management of such cancer it still has dismal outcome. As the progression of HNSCC is complex process which involves interactions between plethoras of factors, so identification of novel prognostic variables could help in adequate management of such type of cancer to improve the prognosis.
In the current study we found that Semaphorin 4D was overexpressed in in 35 (70%) of cases of HNSCC and its expression was correlated with poor clinicopathological criteria like higher grade, advanced stage, presence of lymph node and distant metastases, presence of adjacent foci of CIS, LVI and PNI.
and our results were similar to results of Takada et al. [1] and Derakhshandeh et al. [9] who proved similar results in SCC and Liu et al. [6] who proved that Semaphorin 4D expression is related to poor clinical outcome in cervical cancer patients.
Similarly, Semaphorin 4D was found to be highly expressed in many cancers and its expression was related to poor pathological parameters [1,9] and it was proved to enhance tumor angiogenesis through binding to its receptor Plexin-B1 on endothelial cells which promotes their proliferation which subsequently lead to malignant progression [11,12]. Previous research indicated that Semaphorin 4D has been shown to act directly cancer cells of different organs inducing invasion and metastasis [13,14].
Moreover, Takada et al. [1] demonstrated that Semaphorin 4D increased HNSCC cell proliferation, migration and invasion, which explain our results regarding the association between such marker and progression of HNSCC.
Capparuccia et al. [15] showed that Semaphorin 4D is related to cytoskeleton reorganization and focal adhesion formation which modulate cellular adhesion and motility. Moreover, Semaphorin 4D causes the AKT and MAPK phosphorylation [16,17], the signaling which suppresses apoptosis and promotes malignant cells multiplication, respectively. Taken together, these findings suggest that Semaphorin 4D plays a direct role in progression of HNSCC through the previously mentioned signaling pathways. Takada et al. [1], demonstrate that Semaphorin 4D high expression is present in HNSCCs with bone invasion compared to those without bone invasion. Derakhshandeh et al. [9] study presents a novel role for Semaphorin 4D in modulating the tumor microenvironment by upregulation of ECM collagen formation by fibroblasts, which accompanies tumor progression and facilitates invasion
The dense fibrosis which is rich in collagen type I has been linked with aggressive fatal malignancies and dismal outcome [18-20]. The limitation of the Derakhshandeh et al. [9] study is that it included stage IV malignancy with loco-regional metastases and did not include stage IV with distant metastases, so they did not assessed Semaphorin 4D expression in other stages. In addition, they provided no information regarding smoking history or HPV status was available, leaving their findings more descriptive rather than conclusive. But our study includes different grades, stages and clinical parameters which strengthen our results more. Additionally we assessed the expression of another transcription factor which was incriminated in HNSCC progression; HOXB9.
HOXB9 is a HOX family member, which functions as a transcription factor and it was incriminated in cancer progression. The role of HOXB9 in cancer progression is complicated [21]. HOXB9 was found to be overexpressed in lung and breast cancer, while its expression was decreased in colon, pancreatic and gastric carcinoma [22-24]. In the present study we proved that HoxB9 was overexpressed in 30 (60%) of cases of HNSCC and its expression was correlated with poor clinicopathological criteria like higher grade, advanced stage, presence of lymph node and distant metastases, presence of adjacent foci of CIS, LVI and PNI. which was similar to results of Sun et al. [25] in laryngeal SCC (LSCC) tissues. Xue et al. [5] in oral squamous cell carcinoma (OSCC) tissues and Wan et al. [21] in endometrial carcinoma. Wan et al. [21] found the expression of HOXB9 in endometrial carcinoma is higher than proliferative endometrium and endometrial hyperplasia. In addition, HOXB9 expression in cancer was correlated with higher histological grade and presence of lymph node metastasis which was similar to our results in HNSCC. Sun et al. [25] report that HOXB9 was up regulated in LSCC tissues when compared with the adjacent noncancerous tissues. Moreover they found that a high expression level of HOXB9 was found to be associated with high histological grade and poor prognosis in LSCC. Sun et al. [25] findings provide the first data regarding the association between HOXB9 expression and LSCC progression which is a subtype of HNSCC. Similarly, HOX gene expression is dysregulated in several cancers [25]. But, the expression of different subtypes varies among different types of cancer.
Xue et al. [5] observed that HoxB9 is associated with cancer progression in OSCC and that high levels of HoxB9 are associated with shorter overall survival in OSCC patients. Subsequently, Xue et al. [5] found that knockdown of HoxB9 in OSCC cells decreases cancer cell migration and invasion. This pointed to, the possibility of considering such factor as a targeted therapy for SCC. Effects of HoxB9 in OSCC progression might be due to EMT stimulation through activating the TGF-β1/Smad2/Slug signaling pathway which increased malignant cells invasion and metastases. Although some HOX genes were found to act as oncogenes in solid tumors, others showed down-regulation in other types of cancer [26] and acted as tumor-suppressor genes [27]. The discrepancy between such results may be caused by different roles of HOX genes in different cancer types and the different methods of preparing samples and assessment. Sun et al. [25] have concluded that HOXB9 is a novel biomarker which is responsible for progression of LSCC development and which simulate results other studies that that HOXB9 was frequently overexpressed in many tumors [24,28]. And its high expression was correlated with high tumor grade and poor prognosis [29]. By contrast, decreased HOXB9 expression was found to be related to poor survival in gastric cancer [30].
These findings suggest that protein expression HOXB9 varied according to tumor subtype. However, the underlying mechanism for such discrepancy remains elusive. In a recent study similar to ours in HNSCC, HOXB9 was highly expressed [10]. Several mechanisms could explain the association between high HOXB9 and poor prognosis in cancer one of them is activation of Epithelial-mesenchymal transition (EMT) which comprises a set of changes in the cellular phenotype in which epithelial cells experience a molecular switch from the epithelial phenotype to a highly mobile, mesenchymal phenotype [31]. EMT is mainly observed at the invasive front of tumors and is associated with occurrence of metastasis in tumor progression [32,33]. HoxB9 caused dysregulation of adhesion proteins including E-cadherin, Claudin-1 and Occludin in colon cancer [34]. Additionally, HoxB9 was found to induce EMT in breast cancer by activating the Wnt signaling pathway [28]. These results suggested that over expression of HoxB9 was associated with occurrence of EMT process and cancer progression through activation of TGF-β1/Smad2/Slug signaling pathway [5]. Wan et al. [21] demonstrated that HOXB9 expression is increased in EC patients, and HOXB9 promotes EC cell migration by regulating E2F3 expression. Thus, HOXB9 may represent a prognostic marker and a potential therapeutic target for EC.
CONCLUSION
We found a positive association between Semaphorin 4D and HoxB9 expression in HNSCC and both are associated with progression of the tumor, poor clinicopathological criteria of the patients, that points to the possibility of considering both of them novel therapeutic targets for better management of such serious cancer.
In conclusion, this study confirmed that Semaphorin 4D and HoxB9 expression in HNSCC is increased. This finding supports the possibility of Semaphorin 4D and HoxB9 and/or their associated molecules as targets for anti-metastatic therapy against HNSCC.
LIMITATIONS
The limitations of our study are; small number of samples, lack of follow-up, the retrospective nature of the study and assessment of the markers using single method of evaluation that is the immunohistochemical evaluation of markers so we have recommended performing a prospective study on a large number of patients using genetic methods of evaluation to prove our results and improve the findings.
CONFLICTS OF INTEREST
Authors declared no conflicts of interest.
1. Takada H, Ibaragi S, Eguchi T, Okui T, Obata K, et al. (2017) Semaphorin 4D promotes bone invasion in head and neck squamous cell carcinoma. Int J Oncol 51: 625-632.
2. Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66: 7-30.
3. Lin CC, Fedewa SA, Prickett KK, Higgins KA, Chen AY (2016) Comparative effectiveness of surgical and nonsurgical therapy for advanced laryngeal cancer. Cancer 122: 2845-2856.
4. Busch CJ, Laban S, Knecht R, Hoffmann TK (2016) Immunotherapeutic studies of head and neck tumors: Highlights of the 2016. ASCO Annual Meeting 64: 708-716.
5. Xue M, Zhu FY, Chen LC, Wang K (2017) HoxB9 promotes the migration and invasion via TGF-β1/Smad2/Slug signaling pathway in oral squamous cell carcinoma. Am J Transl Res 9: 1151-1161.
6. Liu H, Yang Y, Xiao J, Yang S, Liu Y, et al. (2014) Semaphorin 4D expression is associated with a poor clinical outcome in cervical cancer patients. Microvasc Res 93: 1-8.
7. Lu Q, Dong N, Wang Q, Yi W, Wang Y, et al. (2013) Increased levels of plasma soluble Sema4D in patients with heart failure. PLoS One 8: e64265.
8. Zhang Y, Wei L, Miron RJ, Shi B, Bian Z (2016) Bone scaffolds loaded with siRNA-Semaphorin4d for the treatment of osteoporosis related bone defects. Sci Rep 6: 26925.
9. Derakhshandeh R, Sanadhya S, Han KL, Chen H, Goloubeva O, et al. (2018) Semaphorin 4D in human head and neck cancer tissue and peripheral blood: A dense fibrotic peri-tumoral stromal phenotype. Oncotarget 9: 11126-11144.
10. Darda L, Hakami F, Morgan R, Murdoch C, Lambert DW, et al. (2015) The role of HOXB9 and miR-196a in head and neck squamous cell carcinoma. PLoS One 10: e0122285.
11. Basile JR, Castilho RM, Williams VP, Gutkind JS (2006) Semaphorin 4D provides a link between axon guidance processes and tumor-induced angiogenesis. Proc Natl Acad Sci U S A 103: 9017-9022.
12. Sakurai A, Doçi CL, Gutkind JS (2012) Semaphorin signaling in angiogenesis, lymphangiogenesis and cancer. Cell Res 22: 23-32.
13. Worzfeld T, Swiercz JM, Looso M, Straub BK, Sivaraj KK, et al. (2012) ErbB-2 signals through Plexin-B1 to promote breast cancer metastasis. J Clin Invest 122: 1296-1305.
14. Ye S, Hao X, Zhou T, Wu M, Wei J, et al. (2010) Plexin-B1 silencing inhibits ovarian cancer cell migration and invasion. BMC Cancer 10: 611.
15. Capparuccia L, Tamagnone L (2009) Semaphorin signaling in cancer cells and in cells of the tumor microenvironment – two sides of a coin. J Cell Sci 122: 1723-1736.
16. Basile JR, Afkhami T, Gutkind JS (2005) Semaphorin 4D/plexin-B1 induces endothelial cell migration through the activation of PYK2, Src and the phosphatidylinositol 3-kinase-Akt pathway. Mol Cell Biol 25: 6889-6898.
17. Aurandt J, Li W, Guan KL (2006) Semaphorin 4D activates the MAPK pathway downstream of plexin-B1. Biochem J 394: 459-464.
18. Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, et al. (2013) Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res 73: 3007-3018.
19. Bagordakis E, Sawazaki-Calone I, Macedo CC, Carnielli CM, de Oliveira CE, et al. (2016) Secretome profiling of oral squamous cell carcinoma associated fibroblasts reveals organization and disassembly of extracellular matrix and collagen metabolic process signatures. Tumor Biol 37: 9045-9057.
20. Fang M, Yuan J, Peng C, Li Y (2014) Collagen as a double edged sword in tumor progression. Tumor Biol 35: 2871-2882.
21. Wan J, Liu W, Feng Q, Liu J and Ming L (2018) HOXB9 promote endometrial cancer progression by targeting E2F3. Death Dis 9: 509.
22. Chang Q, Zhang L, He C, Zhang B, Zhang J et al. (2015) HOXB9 induction of mesenchymal-to-epithelial transition in gastric carcinoma is negatively regulated by its hexapeptide motif. Oncotarget 6: 42838-42853.
23. Wan J, Xu W, Zhan J, Ma J, Li X, et al. (2016) PCAF-mediated acetylation of transcriptional factor HOXB9 suppresses lung adenocarcinoma progression by targeting oncogenic protein JMJD6. Nucleic Acids Res 44: 10662-10675.
24. Shrestha B, Ansari KI, Bhan A, Kasiri S, Hussain I, et al. (2012) Homeodomain-containing protein HOXB9 regulates expression of growth and angiogenic factors, facilitates tumor growth in vitro and is overexpressed in breast cancer tissue. FEBS J 279: 3715-3726.
25. Sun CH, Han C, Wang P, Jin Y, Sun Y, et al. (2017) HOXB9 expression correlates with histological grade and prognosis in LSCC. BioMed Res Int 2017: 1-10.
26. Bhatlekar S, Fields JZ, Boman BM (2014) HOX genes and their role in the development of human cancers. J Mol Med (Berl) 92: 811-823.
27. Wang L, Chen S, Xue M, Zhong J, Wang X, et al. (2012) Homeobox D10 gene, a candidate tumor suppressor, is downregulated through promoter hypermethylation and associated with gastric carcinogenesis. Mol Med 18: 389-400.
28. Hayashida T, Takahashi F, Chiba N, Brachtel E, Takahashi M, et al. (2010) HOXB9, a gene overexpressed in breast cancer, promotes tumorigenicity and lung metastasis. Proc Natl Acad Sci U S A 107: 1100-1105.
29. Seki H, Hayashida T, Jinno H, Hirose S, Sakata M, et al. (2012) HOXB9 expression promoting tumor cell proliferation and angiogenesis is associated with clinical outcomes in breast cancer patients. Ann Surg Oncol 19: 1831-1840.
30. Sha S, Gu Y, Xu B, Hu H, Yang Y, et al. (2013) Decreased expression of HOXB9 is related to poor overall survival in patients with gastric carcinoma. Dig Liver Dis 45: 422-429.
31. Thiery JP (2002) Epithelial-mesenchymal transitions in tumor progression. Nat Rev Cancer 2: 442-454.
32. Ye LY, Chen W, Bai XL, Xu XY, Zhang Q, et al. (2016) Hypoxia-induced epithelial-to-mesenchymal transition in hepatocellular carcinoma induce an immunosuppressive tumor microenvironment to promote metastasis. Cancer Res 76: 818-830.
33. Brabletz T (2012) To differentiate or not--routes towards metastasis. Nat Rev Cancer 12: 425-436.
34. Zhan J, Niu M, Wang P, Zhu X, Li S, et al. (2014) Elevated HOXB9 expression promotes differentiation and predicts a favorable outcome in colon adenocarcinoma patients. Br J Cancer 111: 883-893.
QUICK LINKS
- SUBMIT MANUSCRIPT
- RECOMMEND THE JOURNAL
-
SUBSCRIBE FOR ALERTS
RELATED JOURNALS
- International Journal of Medical and Clinical Imaging (ISSN:2573-1084)
- Journal of Carcinogenesis and Mutagenesis Research (ISSN: 2643-0541)
- Journal of Allergy Research (ISSN:2642-326X)
- Journal of Blood Transfusions and Diseases (ISSN:2641-4023)
- Archive of Obstetrics Gynecology and Reproductive Medicine (ISSN:2640-2297)
- International Journal of Radiography Imaging & Radiation Therapy (ISSN:2642-0392)
- Journal of Cancer Science and Treatment (ISSN:2641-7472)